A Simple Silver Fix May Finally Stop Solid-State Batteries From Cracking
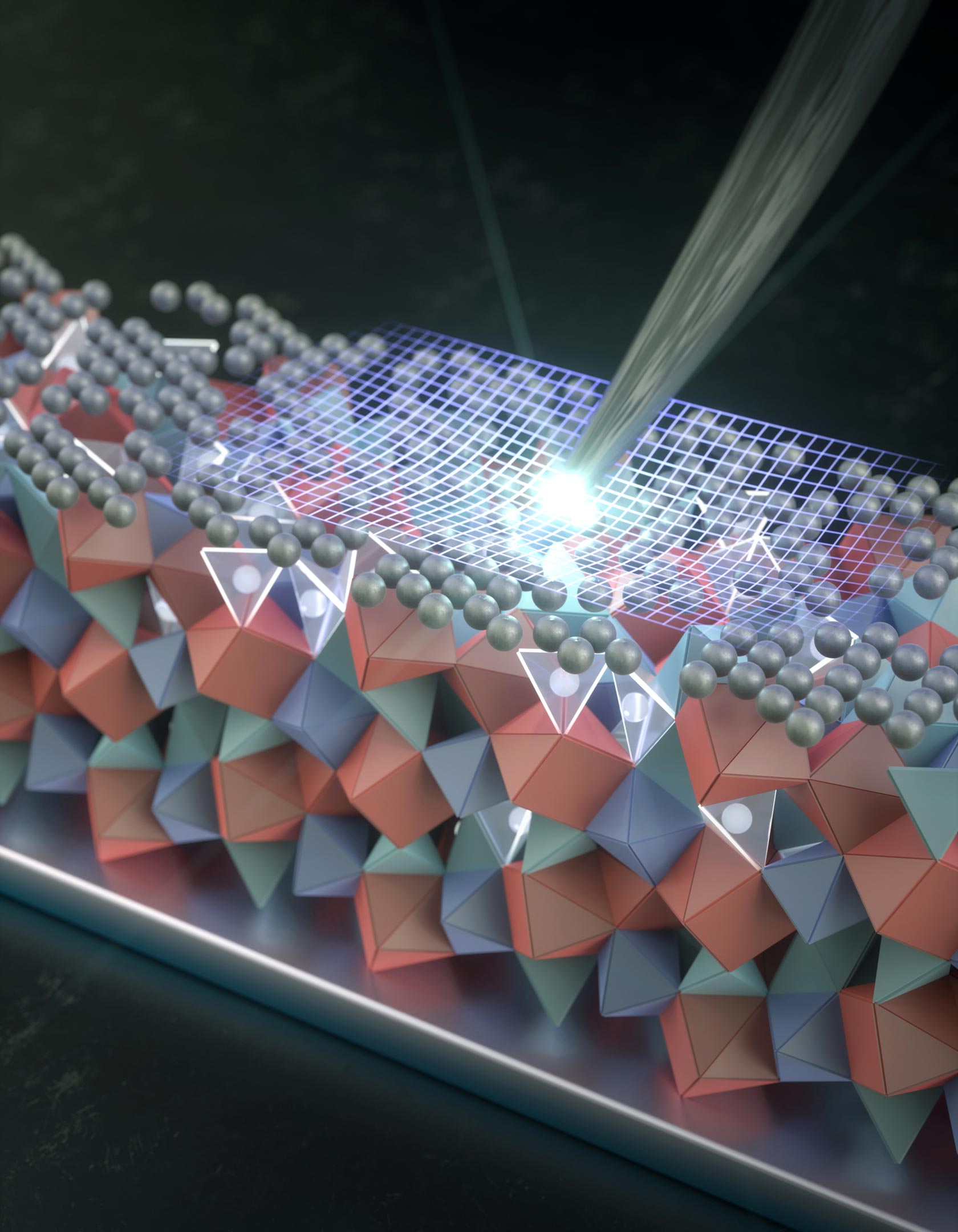
Artist’s rendering of an atomically thin coating of silver and some silver atoms below the surface protecting the crystalline structure of a solid electrolyte for lithium metal batteries in development. Credit: Chaoyang Zhao A nanoscale silver coating could be the key to making ultra-powerful solid-state batteries finally work. Replacing the liquid electrolyte inside today’s batteries with a solid one could unlock a new generation of rechargeable lithium metal batteries. In theory, these batteries would be safer, store far more energy, and recharge much faster than the lithium-ion batteries now in widespread use. Scientists and engineers have been chasing this goal for decades, but progress has been slowed by a persistent flaw. Solid, crystal-based electrolytes tend to develop microscopic cracks that gradually spread during repeated charging and use, eventually causing the battery to fail. A Thin Silver Layer With a Big Impact Building on earlier work published three years ago that revealed how tiny cracks, dents, and surface flaws form and grow, Stanford researchers have now identified a promising solution. By heat-treating an extremely thin layer of silver on the surface of a solid electrolyte, they found that much of this cracking problem can be reduced. As reported in Nature Materials today (January 16), the silver-treated surface became five times more resistant to cracking under mechanical pressure. Just as importantly, the treatment made existing surface defects far less vulnerable to lithium pushing its way inside. This intrusion is especially damaging during fast charging, when nanoscale cracks can widen into deeper channels that permanently disable the battery. “The solid electrolytes that we and others are working on are a kind of ceramic that allows the lithium-ions to shuttle back and forth easily, but it’s brittle,” said Wendy Gu, associate professor of mechanical engineering and a senior author of the study. “On an incredibly small scale, it’s not unlike ceramic plates or bowls you have at home that have tiny cracks on their surfaces.” Gu explained that eliminating every microscopic flaw during manufacturing is unrealistic. “A real-world solid-state battery is made of layers of stacked cathode-electrolyte-anode sheets. Manufacturing these without even the tiniest imperfections would be nearly impossible and very expensive,” she said. “We decided a protective surface may be more realistic, and just a little bit of silver seems to do a pretty good job.” Silver-Lithium Switch Earlier studies by other researchers explored coating the same solid electrolyte material with metallic silver. That material, known as “LLZO” for its combination of lithium, lanthanum, zirconium, and oxygen, was also used in the new work. However, the Stanford team took a different approach. Instead of metallic silver, they used a dissolved form of silver that has lost an electron (Ag+). This charged, dissolved silver behaves very differently from solid metallic silver. According to the researchers, Ag+ ions are directly responsible for strengthening the ceramic structure and making it more resistant to cracking. To apply the treatment, the team deposited a silver layer just 3 nanometers thick onto the surface of LLZO samples and then...
Preview: ~500 words
Continue reading at Scitechdaily
Read Full Article